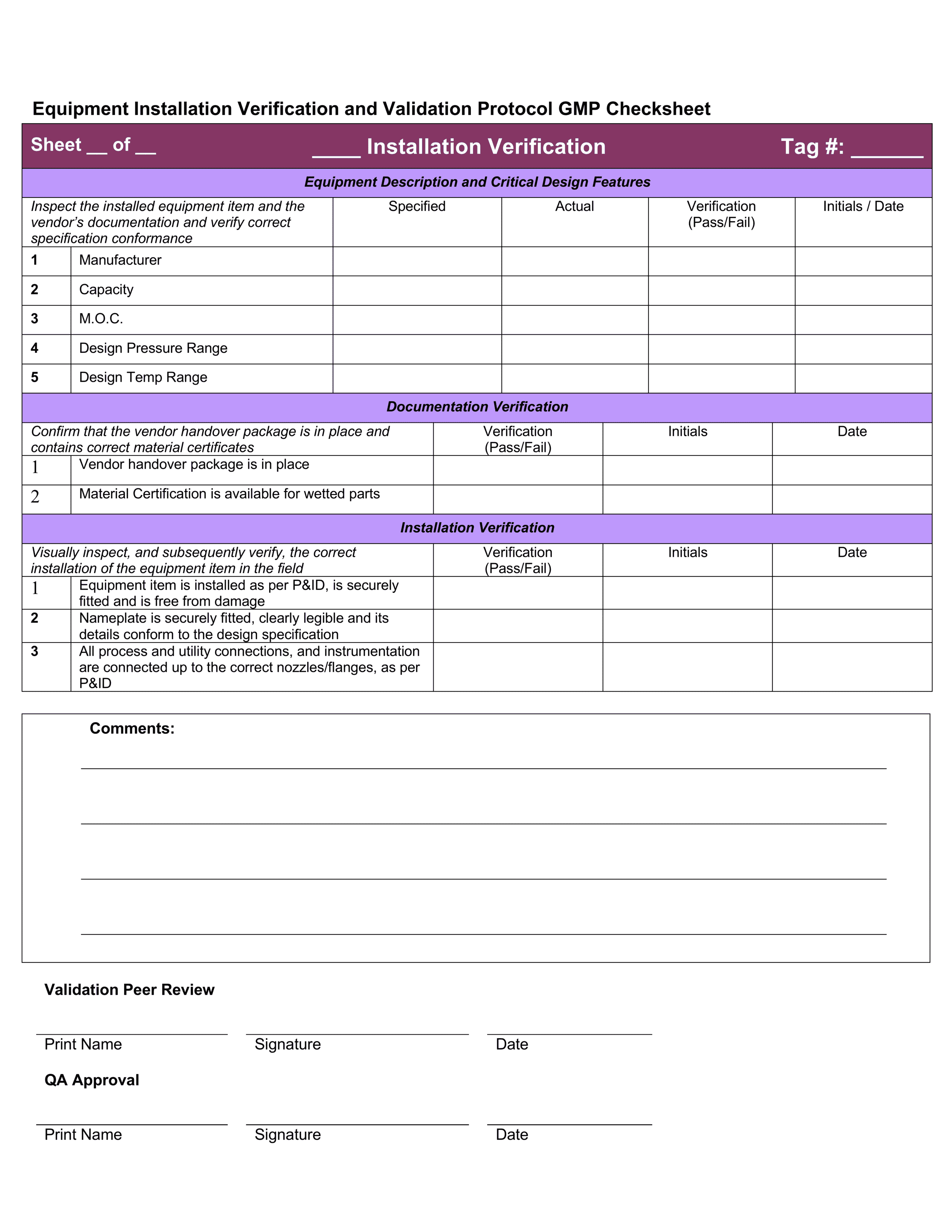
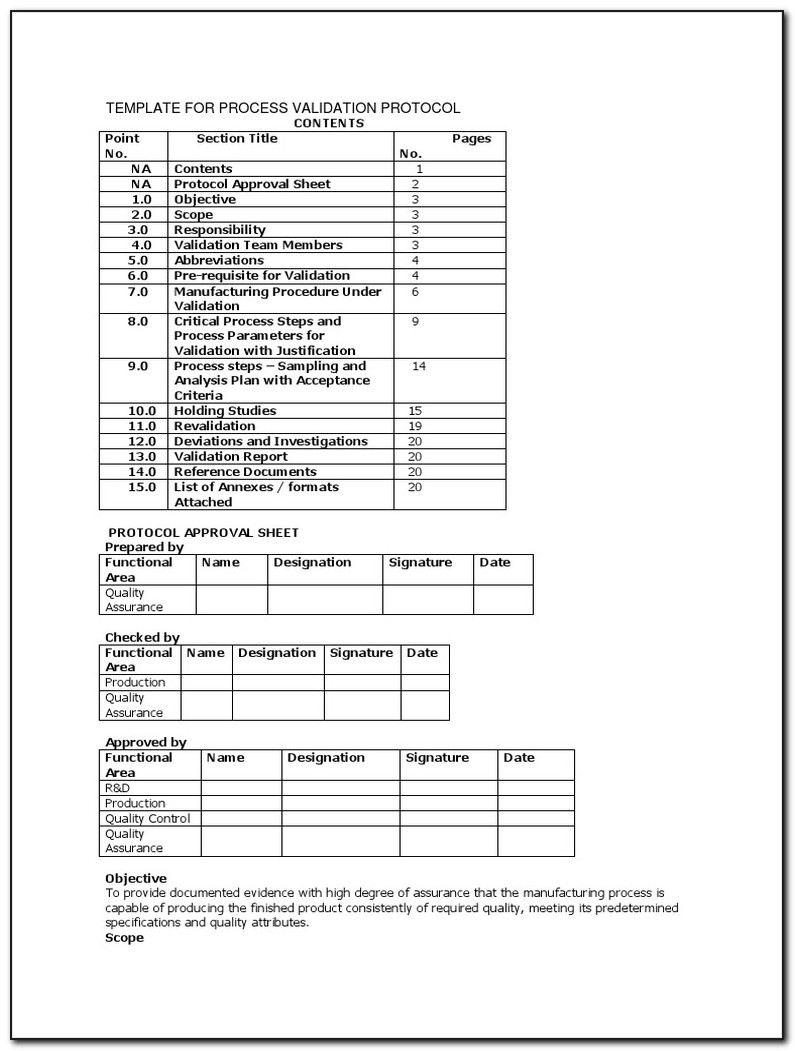
Validation Iq Oq Pq Format. Installation qualification or installation verification testing. IQ OQ PQ represents a way to help ensure reliable performance for equipment for pharmaceutical IQ, OQ and PQ stand for installation qualification, operational qualification and performance Performance qualification protocols and validation should typically include but not be limited to This IQ/OQ/PQ protocol has been reviewed and the contents deemed suitable and approved by the undersigned for implementation The results and final report, where necessary, have been reviewed by the Quality Assurance and Validation Departments and found to satisfy all protocol requirements.

It follows that since all other tests in our protocols are fully written up, if the routine Functional Test Specification (FTS) was of the right format then a recently executed routine or.
Re-qualifications (OQ or OQ/PQ or OQ/IPV)—provide documented verification that the instrument continues to operate as specified by the manufacturer.
This template is suitable for authoring the tests of either User Requirements (PQ) or Functional Requirements (OQ). I just want a few completed validations (IQ, OQ, PQ and reports) to read to give me some ideas on format and cement all the guidence concepts I've read. ideally, if anyone has links to those with that happened to use injection molding to make something, that's best, but even sealers, etc., would be. Installation qualification or installation verification testing.